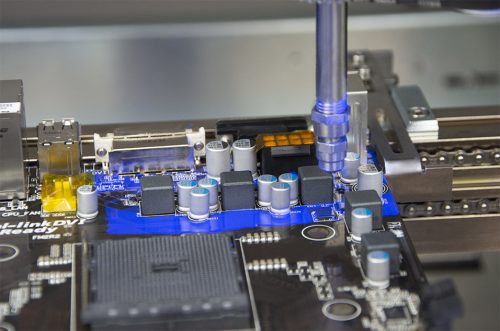
Introduction
Conformal coatings are thin polymeric films, which cover and protect solder joints, the leads of electronic components, exposed traces and other metallized areas on PCBs, from corrosion in their end operating environments. Humidity, condensation, salt-spray, corrosive gases or a combination of all of these elements, activate the start of the corrosion process, which can be accelerated by residues from soldering and other assembly processes prior to coating.
With the increasing adoption of electronics in developing countries, where atmospheric pollution levels are generally higher, there is a growing need for corrosion protection. Additionally, there is an increasing trend towards making smaller devices with greater functionality, resulting in higher density assemblies that are expected to operate trouble free for an extended lifetime, in ever-more aggressive operating environments.
Available Conformal Coating Types
There are many conformal coating chemistries available and each have their own benefits and drawbacks. Conformal Coatings are available in solvent-based, water-based and 100% active materials (nearly everything that is applied in liquid form is converted into solid protective coating), as well as vapour deposited coatings, in which monomeric gases are mixed together in a vacuum, where they are polymerized and deposited onto the surface of the PCB as a protective film.
The primary benefits and disadvantages of the different chemistries available can be summarised as follows:

Coating Standards
Most conformal coatings are either qualified to MIL-I-46058C or meet the requirements of the closely related IPC-CC-830B specifications. In addition they may be recognised by Underwriters Laboratories, either as a permanent coating, in which case the flammability of the coating is assessed to UL94, or as a conformal coating, where the electrical properties will be assessed as part of the UL746E standard.
These tests all require flat coupons to be coated with the conformal coating in question and are subjected to a variety of temperature and humidity conditions to assess the material’s properties. Whilst perfectly acceptable to assess the potential performance of the material, the actual protective capability of the coating in the end use environment is of greater concern to the user. The rest of this article will be devoted to understanding the issues that relate to end use performance in potentially corrosive environments.

Corrosion
Corrosion is a complicated electro-chemical process with a variety of potential mechanisms and causes, well beyond the scope of this article. However, in the vast majority of cases, there are 3 requirements that must be fulfilled in order for corrosion to proceed.
1. Intrinsically electro-chemically dissimilar metals (e.g. Gold/Tin and Silver/Nickel), or the creation of an anode and cathode by application of applied voltage or potential difference.
2. The presence of an ionic species (usually Halides, Hydroxide etc).
3. The presence of mono-layers of condensed water, to dissolve the ionic species resulting in an electrolyte solution.

Fig 1 – required conditions for corrosion of printed circuit board
In order to prevent the possibility of corrosion, it is necessary to remove one of the pre-requisite conditions.
The choice of metals is limited to those used in the solder and solder finish chemistries (which are often dissimilar) and there will always be areas of potential difference in an operating circuit. Cleaning can help remove ionic species, but cannot prevent the re-deposition of ionic species from the operating environment.
Conformal Coatings help prevent the formation of electrolytic solutions by acting as moisture barriers. The coating needs to be a good barrier against moisture and must have good adhesion to the substrate to prevent delamination. Once the coating is delaminated, moisture can eventually collect in this ‘pocket’ and form an electrolytic solution with any pre-existing ionic contamination. This is the reason that cleaning prior to conformal coating is recommended, to provide a powerful synergistic elimination of two of the three pre-requisite conditions for corrosion.
Conformal Coating Application Process
Conformal Coatings have inherent performance criteria specific to each product. However, as we have seen, they function by acting as a barrier to humidity/condensation and the deposition of corrosive species. Therefore, in order to obtain the maximum levels of protection available from the particular chemistry, two key criteria must be fulfilled:
1. The coating must display excellent adhesion to the substrate in question under both dry and highly humid or damp conditions to prevent delamination. This is made more complicated by the potential adverse effects on adhesion of the various residues of modern, ‘no-clean’ assembly technologies, and the importance of the application and curing of the solder resist by the board manufacturer.
2. The coating must fully cover the exposed metal surfaces in order to be effective as a moisture barrier. Exposed leads, (micro)-voids in the coating, such as cracks, bubbles, de-wetted areas etc. will serve as corrosion hot-spots; once the corrosion process starts, it will continue through the metal and potentially beneath the protective coating.
Thus, selecting the ‘correct’ conformal coating is merely the first stage in the protective process. Perhaps of greater importance is the preparation prior to coating (e.g. cleaning) and the actual application process itself.
Conformal coating materials are not intelligent; they go where they are placed (with some naturally occurring wetting/spreading to a greater or lesser-extent, which is a function of the material’s surface tension, thixotropic index and drying time. Most materials will tend to slump away from sharp edges of the component, leads and solder joints due to gravity, and this behaviour can be made worse by longer dry-times and also by baking, if the initial viscosity drop is greater than the increase due to solvent-evaporation. It is also extremely difficult to achieve good coating coverage on the backsides of leads. Understanding and controlling this behaviour, and its effect on the conformal coating coverage, will be key to the performance of coated assemblies operating in harsh environments.
Therefore, the process by which the conformal coatings are applied, and the workmanship and control within that process, will have the greatest influence on the success or failure of that particular coated assembly in surviving its operating lifetime and conditions.
Conclusion
Understanding that the conformal coating can only protect the surfaces to which it is applied with perfect coverage, and that voids, bubbles and cracks in the coating are likely to present a high-probability corrosion initiation site, is a major step towards developing a reliable product.
Choosing a suitable conformal coating chemistry that can withstand the expected environmental challenges whilst still yielding acceptable performance levels is the next step.
But ensuring that the material can be combined with an application process that gives a suitable level of defect-free coverage, is consistent, repeatable and yields the required levels of performance in the end-use environment, is the ultimate step towards ensuring a high-reliability product.